There is a palpable difference between the macroscopic animals we interact with daily and the microscopic unicellular organisms we can only appreciate under a microscope. The transition from unicellularity to multicellularity is perhaps one of the most significant evolutionary transitions that we know of today, even among other large-scale evolutionary transitions, such as tetrapods moving from water to land or the evolution of the gut. At the end of the day, there are limits to how large a single membrane-bound cell can get. The largest of unicellular organisms (that we know of), an alga called Caulerpa taxifolia, can certainly get to macroscopic size, but this alga is still less than 50 centimeters in diameter.

Thus, it seems that unicellular organisms have a hard time growing as large as we multicellular organisms do, that multicellular organisms possess qualities that unicellular organisms do not. As far as we know, there seems to be fundamental genetic differences between unicellular and multicellular organisms. Scientists have previously studied the genomes of some of the closest unicellular relatives to multicellular organisms (such as choanoflagellates) and determined that these unicellular relatives lack certain genes that multicellular organisms use in organizing their cells. However, whether there are structural characteristics that distinguish early multicellular organisms from unicellular organisms is an open question. Ozan Bozdag and collaborators recently provided compelling evidence for biophysical mechanisms that organisms might have used to make the transition from unicellularity to multicellularity.
In the preprint “De novo evolution of macroscopic multicellularity”, Ozan Bozdag and collaborators provide a mechanistic explanation for how unicellular organisms can maintain large multicellular colonies under evolutionary constraints. From a starting sample of baker’s yeast (Saccharomyces cerevisiae), the authors selected for yeast phenotypes that exhibit cell clustering. Essentially, the authors grew baker’s yeast in a liquid medium and looked for clusters of yeast at the bottom of said medium (clusters descend to the bottom of a container more readily than single yeast cells). The single cells, suspended in the liquid medium, were removed from this experiment and fresh medium was added. This process was repeated many times until producing “snowflake yeast”, a type of yeast that has a tendency to cluster together.
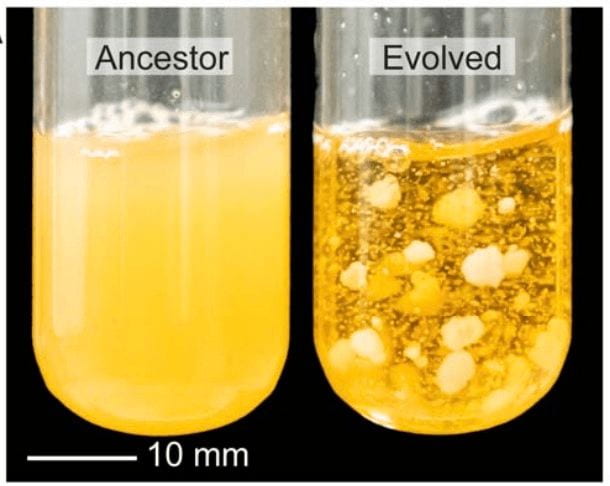
At the end of this process, macroscopic clusters of yeast were observed in the growth medium. This was no small feat for the yeast cells, as individual yeast cells are microscopic and each macroscopic cluster was thousands of times larger than a single cell. When the individual cells in snowflake yeast colonies were examined, their constituent cells differed in structure from normal baker’s yeast: the cells in colonies were significantly more elongated than unicellular yeast cells and entangled together like string.
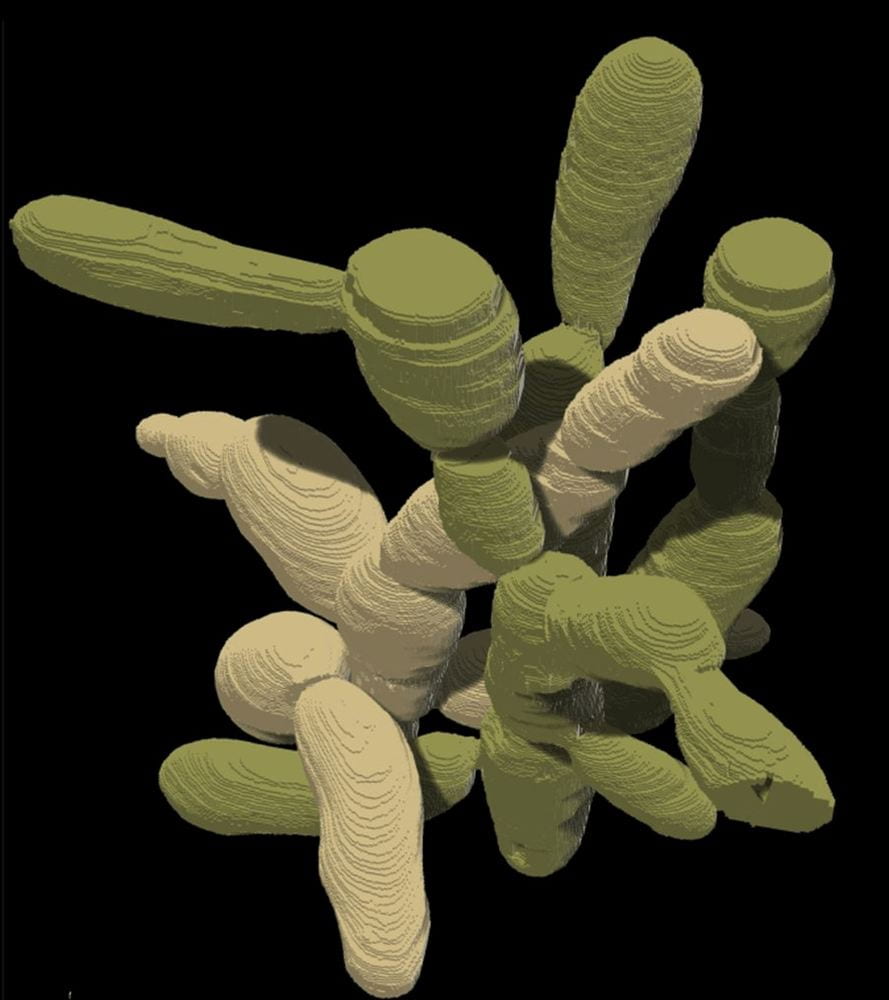
This entanglement provides each snowflake yeast colony with unique physical properties: when subjected to strain, snowflake yeast colonies are significantly more resistant than single yeast cells. To cut cleanly through a colony, one would have to break many more cell membranes because of cellular bonds than if they were tasked with cutting through single cells. As the authors put it, the colonies go from the consistency of a material weaker than gelatin to wood.
Overall, this is a really neat experiment with creative methodologies behind it! It uses a baker’s yeast as a model system because it is 1) unicellular, 2) exhibits a multicellular phenotype when subjected to appropriate selective pressures and 3) has fast generation time, which allows one to observe the process of evolution on relatively short timescales. This study gives us a physical model to imagine what evolution of multicellular organisms might have looked like in the distant past. Further, this experiment demonstrates that cell differentiation is not necessary for the development of stiffness or other emergent properties in cell colonies. In other words, by simulating the process of evolution at an accelerated rate with short-lived cells, we can observe that the selected large cell colonies are composed of structurally different cells than how they started. Furthermore, these colonies have a different stiffness from single cells, meaning that they possess a quality that characterizes them as a distinct unit from simply an aggregation of cells. In short, these colonies are greater than the sum of their parts.
Citations:
Bozdag, G. O., Zamani-Dahaj, S. A., Kahn, P. C., Day, T. C., Tong, K., Balwani, A. H., Dyer, E. L., Yunker, P. J., & Ratcliff, W. C. (2021, January 1). De novo evolution of macroscopic multicellularity. bioRxiv. Retrieved January 5, 2022, from https://www.biorxiv.org/content/10.1101/2021.08.03.454982v1
Ratcliff, W. C., Denison, R. F., Borrello, M., & Travisano, M. (2012, January 31). Experimental evolution of multicellularity. PNAS. Retrieved January 5, 2022, from https://www.pnas.org/content/109/5/1595
Walters, L. J. (2008, December). Ecology and management of the invasive marine macroalga Caulerpa taxifolia. ResearchGate. Retrieved January 5, 2022, from https://www.researchgate.net/publication/227181350_Ecology_and_Management_of_the_Invasive_Marine_Macroalga_Caulerpa_taxifolia
You must be logged in to post a comment.