From “,https://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-12-7”
When you’re faced with weighty decisions like where to attend school or work full-time, you may naturally wonder what the location you will settle down at is really like. Ideally, you’d get to visit the location and see what it’s like for yourself. To ultimately make a good decision, you will need to physically locate and then assess the merits of said environment. For example, I would have never ended up attending school at Amherst if I didn’t think it was a good fit, and especially not if visiting beforehand was an impossibility.
It turns out that marine invertebrate larvae operate a lot like high school graduates becoming college students or new entrants to the job market: they locate and travel to their unique locations to settle down. In a weird way, my decision to attend school at Amherst is somewhat reminiscent of the way in which some anemones, sea urchins, and marine worms learn about their surroundings.
For instance, bottom-dwelling sea anemones start off as tiny mobile larvae called “planulae,” which swim around in search of a suitable home. Once a nice spot is found, they settle down and metamorphose, each forming a polyp that matures and eventually reproduces. Planulae appraise their environment by using a certain organ called the apical sensory organ (ASO). This structure is located at the “aboral” region of the planula larva, or the region opposite to the future mouth of the animal. It also has sensory cells and, in some, a tuft of hair-like structures called cilia. Like antennae, cilia transduce characteristics of the environment into information that the planula can use. In other words, it is the means by which the coral can successfully find suitable locations for starting colonies.
What is particularly shocking about the apical sensory organ is that it is also found in a large range of animals that are evolutionarily distant from sea anemones, like sea cucumbers or annelids. Furthermore, its general structure seems to be shared across these different species, raising fascinating questions in the field of evolutionary studies. Interestingly, the authors of the paper “Larval body patterning and apical organs are conserved in animal evolution” show that the resemblance of different apical organs is anything but superficial. As the “molecular topography” or patterns of transcription factor expression seem to be similar in the animals investigated in said paper, the authors posit that the ancestors of these invertebrates may have also possessed apical organs of their own.
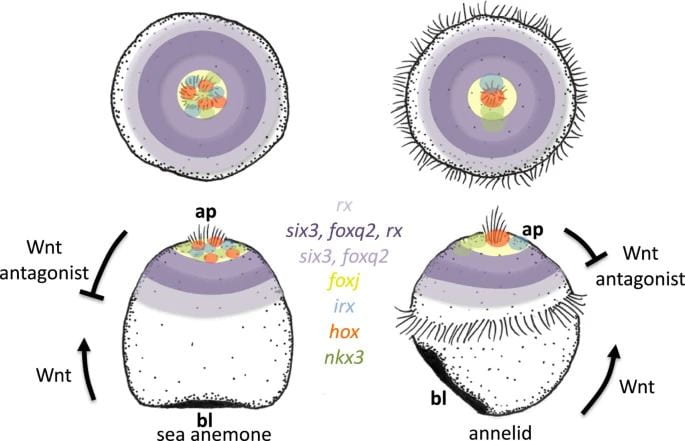
Representatives from two invertebrate groups, cnidarians and lophotrochozoans, that are known to have larvae with apical organs were used to investigate these resemblances more specifically. Cnidarians are members of the phylum Cnidaria, which consists of familiar animals like jellyfish, sea anemones, and coral. Lophotrochozoans are part of a “clade,” which just means that they are descended from a common ancestor. Members of this group include molluscs like snails, segmented worms, flatworms, and more. The representatives that were used were the sea anemone Nematostella vectensis for the cnidarians and the annelid/segmented worm Platynereis dumerilii for the lophotrochozoans.
Using these representatives, the authors ultimately created maps of the top half of the Platynereis and Nematostella embryos and called them “epispheres” (epi- means “above”). The maps prominently feature concentric rings of expression that different genes occupy. For example, the transcription factors six3 and foxq2 are located at the apical poles of the larvae of both Platynereis and Nematostella. These genes are specifically not expressed in the cells composing the poles of the embryos, or those cells that comprise the apical organ. Looking more broadly at these marine larvae, there is a concert of genes arranged in ring-like patterns of expression located at the apical pole that play roles in the formation of the apical organ.
These genes are notably responsive to an evolutionarily ancient form of cell signaling called Wnt signaling, which is responsible for shaping the embryo by establishing its aboral and oral halves. The term “cell signaling” is evocative of its true purpose: cell signaling is a process by which cells communicate, and in this case, it manifests as cells adopting different fates according to their chemical instructions. The authors demonstrate that inhibiting Wnt signaling in Platynereis leads to the expansion or loss of apical organ genes such as six3 and foxq2. This indicates that Wnt signaling has a regulatory role in the formation of the Platynereis apical organ, and mirrors the function of Wnt signaling in the axis establishment in Nematostella.
Taken together, the fact that Nematostella and Platynereis share multiple genes in a concentric ring pattern of expression and both rely on Wnt signaling to pattern their apical ends suggests deep evolutionary conservation of the apical organ. The authors use the central assumption of maximum parsimony to suggest that ancestors of cnidarians and lophotrochozoans could have swum around in prehistoric oceans much like worm and sea anemone larvae do today. This principle is named as such because “parsimonious” is a synonym for “greedy”, which ties into its central idea that is essentially Occam’s razor applied to phylogenetics. In other words, it states that the evolutionary tree with the least amount of changes required to reach a final point is the most likely one. Here, the authors are able to claim that this organ might be evolutionarily conserved because it takes more effort for an animal to construct an apical organ de novo than just possessing and retaining it through evolutionary time.
If the apical organ is so well-conserved, then what exactly does the apical organ do? Across species and phyla, apical sensory organs seem to be composed of flask-shaped cells that secrete neuropeptides, which are intimately linked to the onset of metamorphosis in animals like corals and sea slugs. The authors of the discussed paper identify various types of neurosecretory cells, such as serotonergic cells, and propose that genes involved in neurosecretory cells such as fezf may be evolutionarily conserved elements that contribute to sensing and the onset of metamorphosis. The data presented suggest that the apical organ is intimately related to the nervous system of these marine invertebrates and communicates with the nervous system somehow to regulate the onset of metamorphosis. In particular, despite having no eyes to speak of, a class of light-sensitive proteins called opsins are found in and around the apical organs of both Nematostella and Platynereis. This suggests that the apical organ also initiates phototaxis, or movement in response to light.
The apical organ even has impacts on some marine invertebrate larvae past their metamorphosis. In Platynereis, the authors strikingly show that apical organ cells persist deep in the brain, at the center of a web of nerve fibers with neurosecretory function. Thus, the authors suggest that ancient invertebrates might have gone through gradual metamorphosis, where the apical organ forms the foundation of the nervous system’s organization and therefore its tissue continues to form an important part of the adult’s physiology past metamorphosis.
While hard to spot with the naked eye and superficially unremarkable, the apical sensory organ has a profound evolutionary history and function in the proper development of many invertebrate larvae. This study uses comparative genetics in a brilliant way to demonstrate the similarity of marine invertebrate epispheres and ultimately shines light on the logic of the molecular mechanisms that were present in ancient marine invertebrates.
You must be logged in to post a comment.