Model organisms are non-human species that scientists use to study biological mechanisms. They should be easy to grow, mature quickly, have lots of offspring, and have a sequenced genome. Some of the most popular model organisms are yeast, house mice, fruit flies, worms, and zebrafish. Different organisms have unique traits that make studying specific processes or diseases easier, so choosing the right model for your area of research is important. The Edwards Lab utilizes Dictyostelium discoideum to study how cells move. (For a more in-depth explanation of how my SURF project uses these cells, check out my previous article here!)
Dictyostelium is a single-celled amoeba belonging to the kingdom of protozoa. In general, Dictyostelium is useful for studying cell migration (how cells move), cell differentiation (how cells specialize), phagocytosis (how cells eat), chemotaxis (how cells move in response to a substance), signal transduction (how cells change an outside message to cellular response), and more. Though I use these cells to study macropinocytosis (how cells drink), I think that the most interesting characteristic of this amoeba is in a different stage of its life cycle.
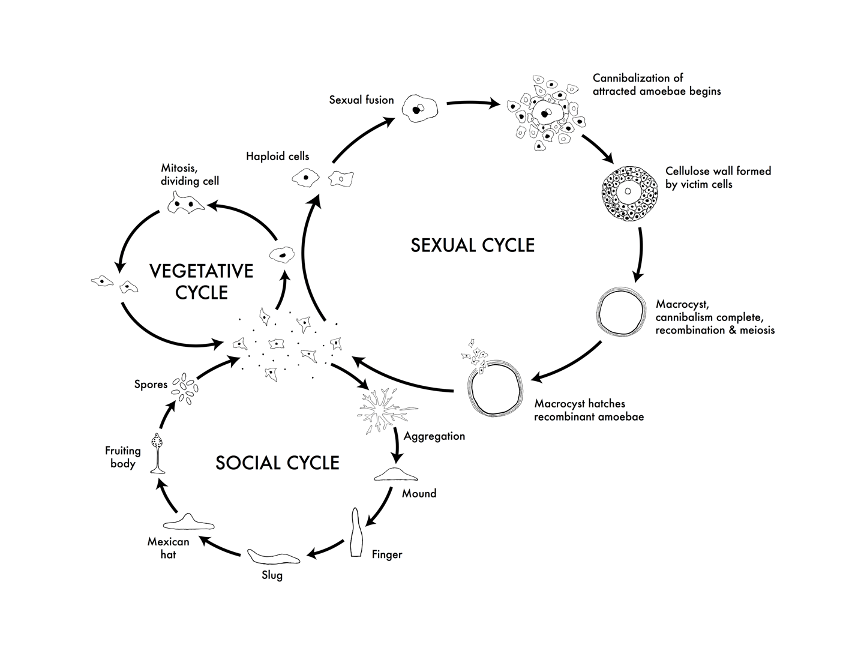
Life cycles of Dictyostelium discoideum. From: http://dictybase.org/tutorial/
Dictyostelium has three different cycles: vegetative, sexual, and social. The cells I use are in the vegetative state, in which they move around in search of food as haploid, single-celled organisms. Haploid means that they have a single set of chromosomes. In the sexual cycle, haploid cells fuse to become diploid cells, meaning that they have two sets of chromosomes each. After the chromosomes recombine to make Dictyostelium with different combinations of genes, the cells split by meiosis into haploid offspring. I find the third cycle, the social cycle, the most intriguing.
The social cycle starts when the cells are starved. The hungry cells all move to a chemical called cAMP and merge to create a multicellular slug. This slug moves toward light and releases spores, or dormant cells. This process allows the cells to disperse into an area that hopefully has more food and better chances of survival. Here’s a video of this happening:
Though Dictyostelium is supposed to be simple to take care of, I’ve had a bit of trouble with them. The cells are not very happy when exposed to bacteria and other debris, so I have to handle all cell-related things under a hood to keep things sterile. A hood, which only my hands can enter, works to filter the air so that contaminants don’t fly onto our culture plates while we’re working with them. Before anything can go inside the hood, it must be sprayed with ethanol. I’m still not used to keeping every little thing sterile or working under the hood, so it takes me a while to use the cells. They’re also incredibly small — you need a microscope to see them. I often wonder if I actually have cells on the wells that I’m doing my experiment on. On multiple occasions, I’ve accidentally sucked off all of the cells during my experiment and ran the entire protocol, only to see no cells under the microscope at the very end. I’ve finally learned how not to do that, but only after a few heartbreaking mishaps. Learning how to work with Dictyostelium has been a trial-and-error process, and I think I’m finally starting to get the hang of it.
If you found Dictyostelium as interesting as I did, you can look up research papers online. Here are a few articles that I found helpful:
The origins and evolution of macropinocytosis
Phagocytosis and Macropinocytosis in Dictyostelium
Living on soup: macropinocytic feeding in amoebae
Dictybase.org has also been a great resource, providing information, videos, and pictures of the cells for free.
You must be logged in to post a comment.