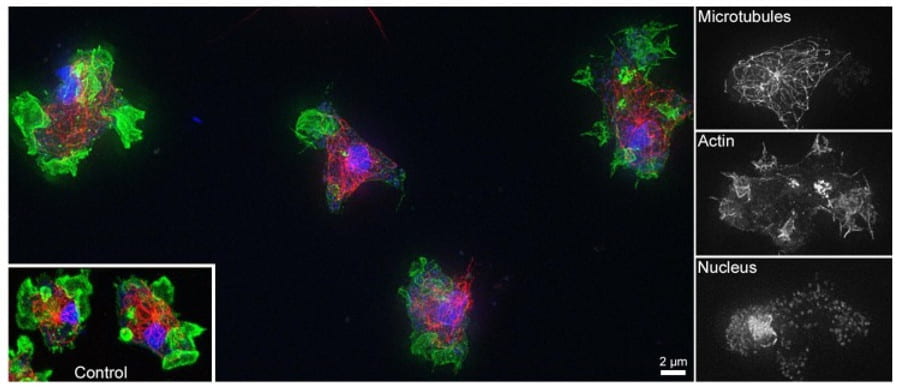
Hello everyone! My name is Grace Lee, and I am a rising sophomore majoring in Biology. This summer, I am conducting cell research through SURF at Amherst in the Edwards Lab. Using the model organism Dictyostelium discoideum, the Edwards Lab studies cell migration. Though the overall goal of the lab is to understand how cells move, my project for this summer will focus on macropinocytosis, which uses similar mechanisms as cell motility, to study a special group of kinases.
Dictyostelium is a type of single-celled amoeba that moves in search of food. When grown in liquid medium, one way that Dictyostelium cells get nutrients is through non-selective fluid uptake, a process known as macropinocytosis. In cells that can grow in liquid media, macropinocytosis accounts for around 90% of the total fluid uptake. The volume of internalized fluid reflects the efficiency of macropinocytosis in cells.
Macropinocytosis results from a signaling pathway that leads to actin polymerization, which shapes the cell membrane into a cup that can wrap around and engulf liquid. An important component in this pathway is a family of phosphatidylinositol 3-kinases (PI3K). Kinases are enzymes that add phosphate groups to molecules. The PI3K family phosphorylates the 3 positions of a ring of phospholipids called phosphatidylinositol (PtdIns). Different PI3Ks add phosphate groups to PtdIns with different combinations of phosphorylated positions. The various PtdIns that result have unique functions in operating macropinocytosis. For example, one PtdIns made by a PI3K is PtdIns (3,4,5)-triphosphate (PIP3). This phospholipid has the 3, 4, and 5 positions of its ring phosphorylated. There are intense patches of PIP3 in the plasma membrane at the start of macropinocytic cup formation, and PIP3 leads to actin polymerization.
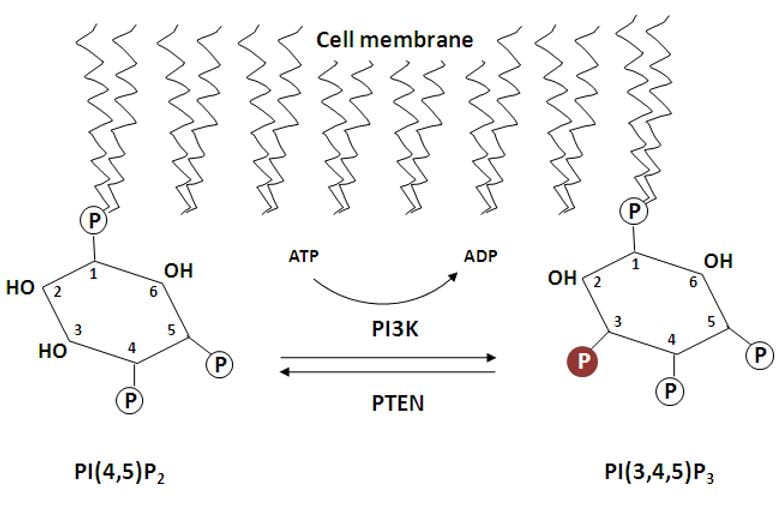
My project focuses on a particular PI3K that we think is important to the later stages of macropinocytosis. By using mutants that lack this particular PI3K, I will be able to observe and record what phenotypic effect knocking out this enzyme has. Currently, I am planning on feeding cells pHrodo-Red dextran, which is a tracer that fluoresces at a low pH. As cells gulp up liquid with pHrodo dextran through macropinocytosis, the dextran will end up in lysosomes for digestion. The low pH of lysosomes will turn on the dextran, and through microscopy, I will be able to see whether my mutants have impaired macropinocytosis. Though this experiment sounds simple, it is an important step to confirming the function of this PI3K. Understanding what this PI3K does will allow us to clarify the big picture of how macropinocytosis and cell migration work.
This summer is a summer of many firsts for me. I’ve never done research or even held a pipette before this summer, so I’m very excited to work in a lab! I’ve also never gone into town, so I expect to roam Amherst and Northampton quite a bit this summer to check out the restaurants. By documenting my summer research experience, I hope to become comfortable with scientific writing and able to explain complicated things in a digestible way. Though I’m a bit nervous about breaking expensive equipment or killing all of the cells, I think that this will be a very enlightening and fruitful summer.
You must be logged in to post a comment.